Swallowing & Communication (Adults)
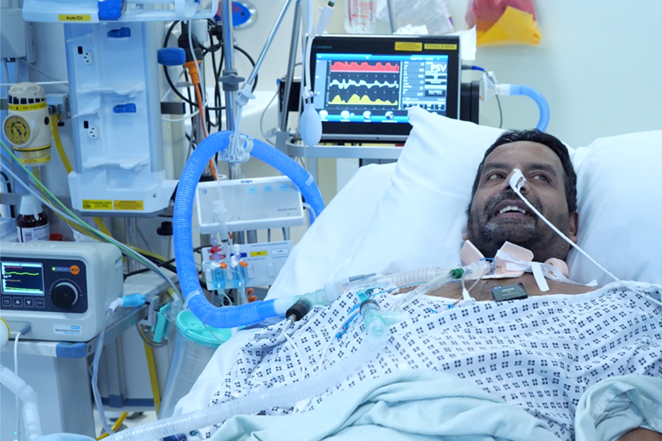
Patients with a new tracheostomy are usually unable to speak: the tracheostomy tube cuff prevents air from flowing through their larynx (voice box). This impacts communication with clinicians, friends and family, which can impede recovery and cause anxiety, especially during recovery from critical illness in ICU. Lack of use of the larynx makes swallowing, coughing, talking and liberation from the ventilator more difficult, and the patient’s recovery process takes longer.
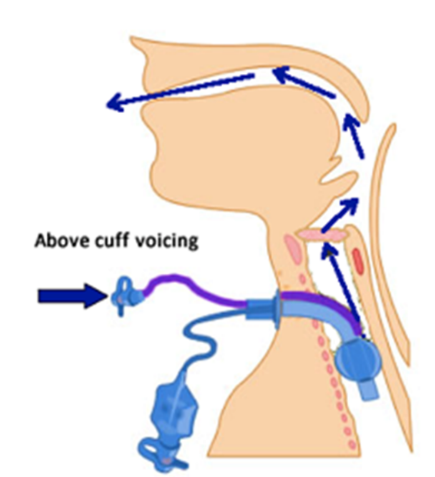
Above Cuff Vocalisation (ACV) allows patients to speak by delivering airflow above the cuff, and out through the upper airway, even whilst the tracheostomy cuff is inflated. You can find out more about standard ACV on our communication pages by clicking here. However, standard ACV uses pipeline medical gas at 4 bar pressure. This approach has no safety features. There is a risk of serious complications such as subcutaneous emphysema and even death if the tube is slightly dislodged.
Our innovation is SEA CTV, Safe and Effective Above Cuff Vocalisation. You can find out more by reading below, downloading the flier, or you can watch this video (Click the link or watch in the player above). We are currently seeking commercial partners for the next step in the development of SEA CTV. See the summary and flier to download on this page. You can watch (and hear!) SEA CTV in action here, and find out more about laryngeal rehabilitation here.
In 2018, a £595k National Institute of Health Research (NIHR) Invention for Innovation (i4i) grant was awarded to Manchester University NHS Foundation Trust (MFT) to create and test a new ACV device with safety features. The device is independent of lung ventilation and delivers ACV only in expiration (to mimic normal speech). The project concluded in December 2024. Co-developed with patients, clinicians, academics and engineers from Manchester Metropolitan University, the device is at Technology Readiness Level (TRL) 6: technology model or prototype demonstration in a relevant environment.
Initial laboratory and isolated animal-part testing determined optimum parameters for safe and effective ACV (e.g. flow rate, pressure, humidity, temperature and safety features). The device has undergone clinical investigation in a randomized-controlled trial at MFT testing safety, tolerability, effectiveness and the impact on the patient journey (including healthcare economic analysis). Data collection completed in December 2024 and we will report the outcomes of the study in full during 2025. The headlines are that SEA CTV gave patients a voice without any complications reported during the trial. We evaluated swallow in detail and demonstrated a significant improvement in swallow function and laryngeal sensitivity after SEACTV use, with associated reductions in anxiety. We will post the full results after they have been published in a peer reviewed journal.