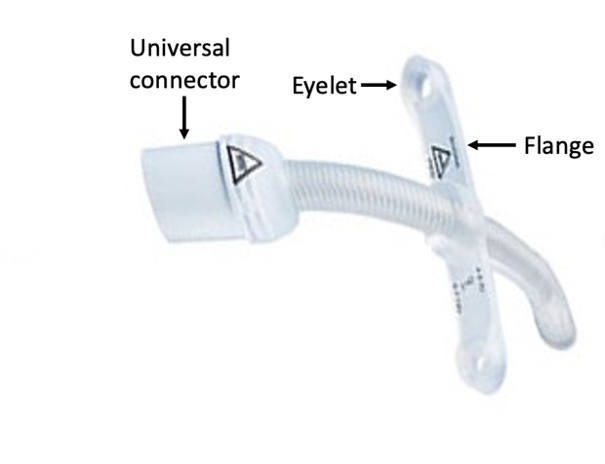
A series of incidents has been reported, involving Bivona Silicone tracheotomy tubes. The are reports of some tubes breaking at key points:
- Eyelet of the flange
- Base of the flange
- Junction with the universal connector
Supply problems have meant that some tubes are being used beyond their intended lifespan in some instances. The NTSP has worked with key stakeholders and the national team and issued the following safety notification and advice. The notice can be downloaded from the documents section of the NTSP website and will be distributed by relevant stakeholders. Based on analysis of the incidents reviewed (and until any further information becomes available), this safety notification is applied to all silicone Bivona cuffed and cuffless tubes.
ACTIONS TO BE TAKEN:
· Apply increased vigilance in all tracheostomy tube care
· Check all visible parts of the tracheostomy tube and its connectors for any signs of wear or breakage on at least a daily basis
· Record the batch and lot number for all tracheostomy tubes used
· When the tracheostomy tube is secured, twill fabric tape should be in contact with the tube eyelets, not Velcro
· Clean and reuse tracheostomy tubes only as per manufacturers’ guidance
· Replace any tracheostomy tube that is damaged or showing signs of wear
· Keep any damaged or degraded tracheostomy tube for subsequent review
· Escalate any concerns or issues with a tracheostomy tube to usual tracheostomy-related healthcare point of contact
· For healthcare professionals: in the event of a problem, as appropriate submit
- A clinical incident report
- MHRA Yellow Card form: https://yellowcard.mhra.gov.uk
- NHS Supply Chain product complaint: https://www.supplychain.nhs.uk/contact/submit-product-complaint/
And email ICU Medical Customer Service: ukcs@smiths-medical.com